Question
A complex with maximum spin angular momentum
Options :
[FeF6]3–
[Fe(CN)6]3–
[Fe(H2O)6]2+
[V(H2O)6]2+
Show Answer
Answer :
[FeF6]3–
Solution :
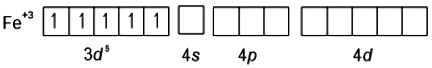
F⊝ with Fe+3 behaves as WFL, Hence pairing does not take place, so it forms high spin complex.
[FeF6]3– ⇒ sp3d2 hybridisation
Number of unpaired electron = 5 [Fe(CN)6]3- ⇒ d2sp3 hybridisation
Fe+3 = 3d5 Number of unpaired electron = 1
[Fe(H2O)6]2+ ⇒ sp3d2
Fe+2 = 3d6
Number of unpaired electron = 4
[V(H2O)6]+2 ⇒ d2sp3 hybridisation
V+2 = 3d3
Number of unpaired electron = 3
Spin angular momentum =
S = total spin quantum no.
More the number of unpaired electron more will be spin angular momentum. [FeF6]3– has 5 unpaired electron hence maximum spin angular momentum value.
Report
More Similar Tests